Features & Columns
Eat My Phone
 TESTING...: Elizabeth Skovran's lab at San Jose State University is refining a process by which bacteria eat rare-earth metals to allow for efficient and economically viable recycling. Photo by Harry Who
TESTING...: Elizabeth Skovran's lab at San Jose State University is refining a process by which bacteria eat rare-earth metals to allow for efficient and economically viable recycling. Photo by Harry WhoThere is a blender in the corner of the Skovran biology lab on the fifth floor of Duncan Hall at San Jose State University. It's similar to the kind found in kitchens everywhere, only this one's not for making pesto.
The brand name is Blendtec, made quasi-famous by a series of cheeky YouTube videos known collectively as "Will It Blend?," in which a white-lab-coated technician tosses golf balls, cigarette lighters and even an Amazon Echo into the appliance. (Spoiler alert: They always blend.)
The blender at SJSU operates in that same spirit—except in Elizabeth Skovran's lab, they aren't looking for likes and lulz. Instead, Skovran and her team are chopping up electronics in an effort to help the environment and potentially launch a very lucrative business.
Every few months, Skovran and her students get out the old Blendtec and toss several used smartphones into it. Using a fume hood to deal with the toxicity of the task, they reduce their mobile handsets to a fine black powder. That pulverized cellphone stuff is then put into a medium with a culture of bacteria—specifically, a subset known as "methylotrophs." To the untrained eye it might appear that Skovran and her team are competing to create the Worst Smoothie Ever. In fact, they are refining a process that could revolutionize the electronics industry.
Skovran and her collaborator, Michigan State biochemist N. Cecelia Martinez-Gomez, are looking for a solution to an ominous worldwide problem. They aim to develop a method for safely, cleanly, cheaply and efficiently recycling rare earth elements (REEs).
REEs are an essential material in the tech industry. The list of technologies that would not be possible without the 17 metals classified as REEs is enormous: Smart phones, batteries, satellites, GPS equipment, electric-vehicle engines, computer hard drives, wind turbines, X-ray machines, dental implants, lasers, LED lights, loudspeakers, even military weaponry.
"They are vital," says microbiologist David Reed of the US Dept. of Energy's Idaho National Laboratory, the nation's leading facility for nuclear and other materials research. "We would not have the world we live in today without them."
What's more, these metals are just as crucial to the world of tomorrow. "They're pretty much critical for all green technology," says biologist and researcher Vicki Thompson, also at the INL. "Wind power uses them for its magnets. Electric cars use them for batteries. They're involved in most of our lithium-ion (rechargable) batteries."
The problem is that these materials can only be obtained via environmentally devastating mining practices, and China controls about 80 percent of the world's production of these elements. The United States has exactly one mine for these materials. The Mountain Pass Mine in the Southern California desert, which has already been shut down twice, is preparing to ramp up production to offset rising tariffs on Chinese REEs. Anticipating big disruptions to the REE market because of Chinese domination and the Trump administration's trade war, a company called UCore is exploring opening a new mine in Alaska.
These materials are notoriously difficult to recycle and the vast majority of them—95 percent according to some reports—end up in landfills. So, the race is on to find a way to effectively recycle REEs, and Skovran thinks she might have the answer.
Scientists have been studying the unique properties of methylotrophic bacteria for decades, but only in recent years was it discovered that these bacteria—which are commonly found in lakes, in soil, even on the leaves on plants—have the ability to extract rare earth metals from raw ore and post-consumer electronic garbage. They can even pull REEs from industrial waste byproducts, such as "fly ash," a coal product, and "red mud," a waste product of aluminum production.
The bacteria convert these waste metals into inorganic crystals within their cells by emitting chemicals that bind with rare earth metals, then drawing them back into their cells and consolidating them into crystals. The bacteria use methanol as their primary food source, and the crystals made up of REEs act as enzymes that they use to digest their food.
 LEARNING EXPERIENCE: Elizabeth Skovran with a graduate student in her SJSU lab. Skovran enjoys working with graduate and undergraduate students. Photo by Harry Who
LEARNING EXPERIENCE: Elizabeth Skovran with a graduate student in her SJSU lab. Skovran enjoys working with graduate and undergraduate students. Photo by Harry WhoThe Skovran team is working to figure out how to stimulate that process by making the bacteria hungrier for REE-laden waste. That way, the organisms can do the heavy lifting of separating the rare earth wheat from the industrial waste chaff, and make reclaiming REEs commercially viable.
Eating It Up
Contrary to their name, rare earth elements are not all that rare. They are found everywhere.
However, mining REEs is only feasible in places where there are large concentrations of them. Even in those cases, REE mining involves a complex process to separate and purify the metals found in ore. This process includes bathing the ore in acid and washing it with solvents. One of this process' byproducts is radioactive sludge. Regions with REE mines in China—a country notorious for lax environmental regulations and low labor costs—have been devastated by crop failures, animal illness and widespread cancer.
The environmental consequences of mining, along with the Trump administration's trade policies, have exerted enormous pressures on finding ways to recycle REEs. Currently, however, that process is time-consuming, prohibitively expensive and highly toxic. "If you open up a computer hard drive and you want to get the magnet out that contains these rare earth metals," Skovran says, "it takes considerable time to break into all these electronic components and grab the parts from the computer or cellphone that has these metals." As a result, rare earths are recycled at a rate of about 1 percent. And even if recycled, they still generate waste.
"They usually ship them out of the country, to China or Africa," Skovran says of thrown-away cell phones and electronics. "And they usually sit in big piles in landfills. There is a lot of nasty stuff in your cell phones—brominated flame retardants, mercury, cadmium—lots of bad stuff. Waste accumulates over time, and as things break down, it could leach into and poison the water supply."
The push to find new ways to recover these REEs from electronic waste and other waste streams has intensified in recent years. "Five years ago," Reed says, "there was very little [effort to find new recycling methods]. You'd occasionally see a paper on it. But now, it's become obvious that it's something we really need to address."
There are other companies and research units working on the problem, but the Skovran approach may be unique. "We are in the discovery mode now," says Martinez-Gomez, Skovran's Michigan State collaborator. "We are literally learning something new every week."
Skovran and Martinez-Gomez have been pursuing the bacterial method for four years now. In that time, they've learned that methylotrophic bacteria release a molecule, called a "chelator," that binds to the rare earth materials and then absorbs it back into the cell, creating a tiny inorganic crystal of pure metal within the bacterial cell. "Our separation process is pretty efficient," Martinez-Gomez says. "The microbe is doing all the work for us."
Skovran shows me a collection of petri dishes, filled with black powder (our iPhone smoothie) dotted with tiny pink polyps. Those are the methylotrophic bacteria.
There are still problems to crack with this approach. Though the bacteria are good at drawing out the REEs and purifying them, they don't do it at scale. Like an animal feeding at a trough, the bacteria reach a saturation point and stop absorbing the metals. The Skovran team is attempting to engineer the bacteria to keep them working past their "full" point. (An alternative avenue would be to take the bacteria out of the loop completely and somehow isolate the molecular chelator they release to attract the metals.)
Another problem: The organic solution to extracting REEs doesn't do anything about the remaining waste material. "If you were to blend up a cellphone," Skovran says, "and put that in a solution, it'll absorb [the REEs], but what about the remaining toxicants left behind in the cell phone? What'll you do with that?"
One answer, Skovran believes, lies in fly ash, a fine powder that is a byproduct of coal power plants that is used in the production of cement. Fly ash has valuable stores of REEs. Once those metals are extracted, the material can still be used for cement, creating, at least theoretically, zero waste.
"[Post-consumer] electronics is sexy to talk about," Skovran says. "But in reality, if you really wanted to avoid that toxic waste, blending up all these waste electronics would just release more toxic products."
Rare Discovery
The discovery of the process by which methylotrophic bacteria use REEs is less than a decade old. Researchers had isolated a particular gene which worked as an enzyme, allowing the bacteria to consume methanol as its primary food source. But another similar gene was discovered in the bacteria's DNA that resisted anyone's efforts to figure out its purpose.
Skovran was working in her post-doc program at the University of Washington, contributing to the investigation of these bacteria and the mystery gene. "The gene was known about for about 20 years," she says. "But you delete the gene from the cell and there was no effect."
The connection between the bacteria and rare earth metals eventually came from the metals side of the equation. Researchers at Gifu University in Japan were studying the effects that REEs had on micro-organisms associated with plants. It was then they discovered that methylotrophic bacteria needed rare earth metals to digest their main food source.
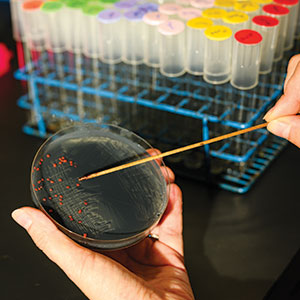 TASTY DISH: A pitri dish containing finely ground electronics dust and tiny blobs of methylotropic bacteria, which consume rare-earth elements. Photo by Harry Who
TASTY DISH: A pitri dish containing finely ground electronics dust and tiny blobs of methylotropic bacteria, which consume rare-earth elements. Photo by Harry WhoThough she was steeped in the workings of the bacteria, Skovran at that time knew almost nothing about REEs. "I had to look at the periodic table to figure out where they were," she says. Still, she said, everything changed in the outlook for her research after that.
"When that (Japanese) paper was published, I had an aha moment for sure. I thought, 'Wow, this is a great new addition to the field of biology in general.' Nobody thought these rare earth metals had any physiological or inherent role in biology. And this proved that they did."
In the rarefied world of methylotrophic bacteria, the Gifu discovery qualified as a bombshell, Skovran says. "In fact, it was a little disbelieved at first, because it was so baffling. There was a lot of pushback in the field. But the more and more the scientific community started accepting that, yep, they actually do these things, then the field got more exciting. We started researching how they do that, and this idea came into play: Let's take this process they do naturally and turn it into something valuable to society."
Microbio Mentor
Skovran landed her faculty position at San Jose State shortly after the discovery. But, she says, the two events were largely incidental. She came to SJSU because she was intent on working with undergraduates. Major US universities are largely divided between research universities offering doctorate degrees (often referred to as "R1" universities) and primarily undergraduate institutions (PUIs).
As an undergrad at the University of Wisconsin-La Crosse, Skovran had a rewarding experience under the tutelage of an inspiring teacher, and it was important to her to follow that template as a teacher and a researcher.
"I had a professor that I looked up to a lot, and I wanted to be that kind of professor. It was always on my mind. I wanted to have a similar kind of job where I worked with undergraduate students and could facilitate their careers by enabling them in research."
At SJSU, Skovran works with a couple of dozen students, most of them undergrads (there are a few master's degree students in the program as well). Some of those students, she says, are primary authors on some of the papers that have been published out of the lab's research, an invaluable experience for undergrads.
"It opens a lot of doors," she says. "A lot of my undergrads have gone on to top PhD programs after they graduate, or get snapped up by the biotech industry, especially because they have their names as first author on publications."
Bacterial Business
Within the next few years, the team's ambitions are to develop a platform that is both affordable and efficient for extracting REEs from commercial waste. "What route do we take at that point?" Skovran asks. "Do we want to turn this into a company? Or do we want to focus on making cool discoveries and maybe partner with someone? I don't know."
Quietly but urgently, governments, industry and researchers are looking for ways out of an unsustainable situation. For example, geologists in China have recently discovered a way to isolate the geological characteristics that make REEs abundant in that country. In theory it might be possible to artificially engineer rare earth materials—similar to the way way jewelers create diamonds in the lab. The Trump administration is working with the Australian government to create a more stable source of REEs without China. The US Department of Energy's Critical Materials Institute in Ames, Iowa and the Oak Ridge National Laboratory are among those focused on production and processing of REEs.
"We work at a national lab for the department of energy," says Reed of the INL. "Our focus is how do we make energy sources for the future? Companies may be looking down the road 10, 20, 30 years—how can we make money from this? Our goal is to look at [recycling efforts] and figure out, can it be scaled? That's why we're doing techno-economics, life-cycle analyses, lab-based scaling. Before a company is going to do a full-scale investment, you want to show a technology can work."
One thing everyone agrees on is that rare earth elements will remain vital to a growing economy and a technologically advanced future. And there's no plausible replacement for REEs on anyone's horizon.
"The demand is high," said Martinez-Gomez, "and it's only going to get higher."